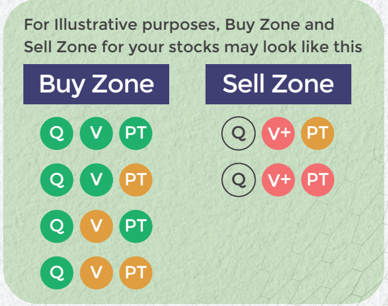
Stocks - Q : Quality | V : Valuation | PT : Price Trend
Funds - P : Performance | Q : Quality
Funds - P : Performance | Q : Quality
Lupin has launched the first generic version of Pred Forte (Prednisolone Acetate) Ophthalmic Suspension USP, 1% in the United States. Being the first generic to be approved and launched, the company is entitled to 180-day competitive generic therapy (CGT) exclusivity.
Prednisolone Acetate Ophthalmic Suspension USP, 1% is a generic equivalent of Pred Forte Ophthalmic Suspension, 1% of AbbVie, Inc. and is indicated for the treatment of steroid-responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe. Pred Forte had estimated annual sales of $198 million in the U.S. (IQVIA MAT August 2024).
Lupin is an innovation led transnational pharmaceutical company producing, developing and marketing a wide range of branded and generic formulations, biotechnology products and active pharmaceutical ingredients (APIs) globally.
| Company Name | CMP |
|---|---|
| Sun Pharma Inds. | 1808.50 |
| Dr. Reddys Lab | 1342.45 |
| Cipla | 1472.45 |
| Lupin | 2147.55 |
| Zydus Lifesciences | 973.90 |
| View more.. | |

MoneyWorks4Me rating and ranking of funds for SIP is available to subscribers only. Moneyworks4Me is not a rating and ranking agency, however it is required that users have a way of selecting funds and building a Portfolio. The method used by it are described below to enable users to understand the logic behind the rating and ranking Subscriber will find more details on this in the various content made available from time to time. In case you need more please write to besafe@moneyworks4Me.com
MoneyWorks4Me rates and ranks mutual funds based on the following data-driven system:
Funds ranking in screeners: Performance Consistency and Quality are two parameters used for ranking funds for SIP. The ranking as follows GG, GO, GR, OG, OO, OR, RG, RO and RR.
With the same color-coded funds, the one with the higher Average 3-year rolling returns (over 5 to 10 years), the number that appears in the Performance tag, ranks higher.
Here is the summary:
The third tag Upside Potential is not relevant for SIP. It is relevant for lumpsum investments in Mutual Funds.

A 5-Step Journey to Financial Freedom
Read this Book for Free.*This Offer is only valid till this month only. Claim it now.
Read NowDid you know that market corrections can actually present great opportunities to buy high-quality stocks at discounted prices? By taking advantage of these times of volatility, you can position your portfolio for long-term growth.
At MoneyWorks4me Portfolio Advisory, we specialize in helping investors navigate market fluctuations and build a strong, diversified portfolio. With our collaborative approach, you can maintain control over your investments while benefiting from our expertise and guidance.
If you're interested in learning more and with a minimum portfolio size of 25 L+, we can help you manage your portfolio, no matter the size. let's connect and discuss how we can work together. And as a bonus, we're offering a FREE Portfolio Review using our "Portfolio Manager" tool during our conversation.
So why wait?
Let's get started today and take your portfolio to the next level!
#Limited slots available.
Do you want to Invest in Undervalued Handpicked stocks and earn high Returns?
Winning and long lasting portfolio is made of Quality Stocks, but how simple is that?

As an Investor most important decision making questions are?
Make an informed decision for Stocks
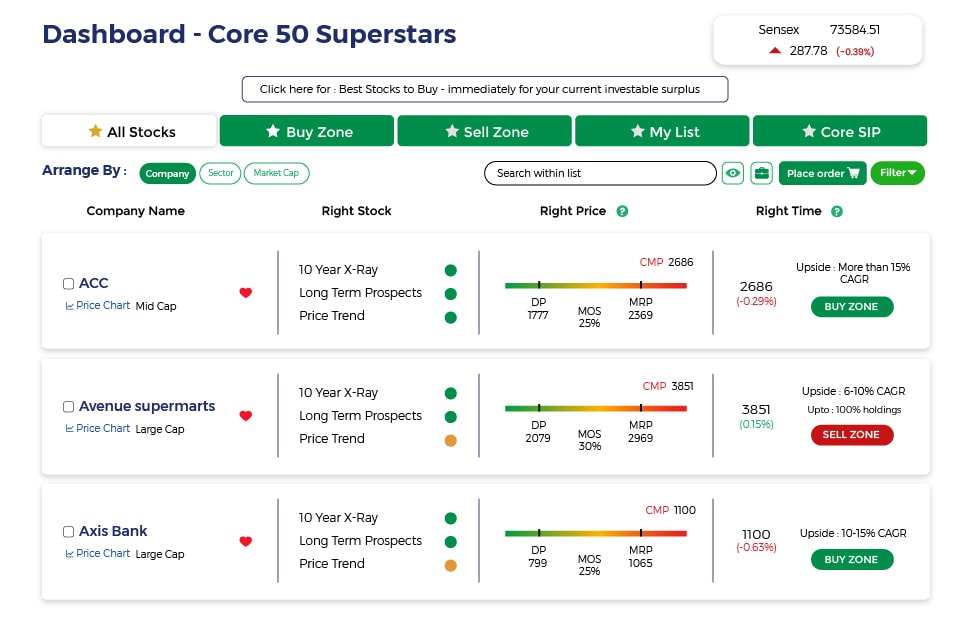
Invest using an intelligent system with powerful data-driven tools that help you identify opportunities and make informed buy-hold-sell decisions
Buy quality Stocks when they are available at reasonable prices and supported by an upward price trend and Sell when they are Overvalued using the Decizen Rating System. Covers 3500+ stocks
Make an informed decision for Funds
*Color code for outperformance consistency
*Number is average 3 year rolling returns

Want to invest successfully in stocks?
How the heck do you select a solution that ensures it?
Check how good is your current solution!Does it get you focused on meeting your financial goals?

Does it get you focused on meeting your financial goals?
Investing is to means to funding your goals. Your solution must help you get clarity of your goals and how you should invest to reach them. Does your solution include Financial Planning?
Does it have a way of investing that you are confident will work for you?
Does it have a way of investing that you are confident will work for you?
Are you clear of the rationale behind recommendations?
Do you have a way of answering the most important questions: Which stocks are worth investing in, at what price and how do you build your portfolio?

Does it make it easy to find opportunities without wasting your time?

Does it make it easy to find opportunities without wasting your time?
Does it share the tools to enable decision making transparently?
Does it share the tools to enable decision making transparently?
Finally stock investing is all about make informed decisions.
Does your solution provide easy-to-use decision enabling data, information and tools?

Does it help you become a better investor

Does it help you become a better investor




Limited to the first 10 users today!

Practical Stock Investing Hand Book
By - Raymond Moses
Founder of MoneyWorks4Me | Consultant | Trainer | Author | TEDx Speaker
Register & Get Instant Access!Don't miss out! First 10 users today get it FREE

With this 4 hrs Masterclass, you’ll be able to:
Take control of your financial future and start building your ideal portfolio today
- with reliable research at your fingertips.
Unlock the Power of Core Superstars Now →