This is Part 2 of our series on the CDMO Industry. In case you missed Part 1, Click Here.
The obstacles of one sector can become the stepping stones for opportunity in another.
The increasing cost of research and development and getting a drug approved by the FDA is not sustainable, especially now as regulatory and legislative issues are at the forefront of debate. This problem requires a sharing of risk and reward in manner that reduces the financial burden of innovators. CDMOs provide the perfect opportunity to aid innovation in this context. CDMOs can aid innovators through: (a) cost efficiency and expertise, (b) flexibility and scalability, and (c) risk mitigation.
Cost Efficiency and Expertise
CDMOs specialize in certain areas of drug development and manufacturing, allowing them to operate more efficiently than pharmaceutical companies attempting to handle all processes in-house. This specialization enables CDMOs to provide high-quality services at a lower cost, making them attractive partners for pharmaceutical firms looking to manage their R&D budgets effectively. CDMOs invest in the latest technologies and equipment to stay competitive. Pharmaceutical companies can access these advanced capabilities without the need for substantial capital investment, allowing them to leverage cutting-edge solutions to enhance their R&D efforts.
Flexibility and Scalability
Pharmaceutical companies facing high R&D costs benefit from the flexibility and scalability that CDMOs offer. CDMOs can quickly scale up or down based on the needs of their clients, accommodating varying demands without the pharmaceutical company having to invest in expensive infrastructure and equipment. This flexibility helps pharmaceutical companies manage their resources better and respond swiftly to market changes. The capacities of CDMO players are fungible, i.e. the capacity once used for a particular drug or client can be used for another client or drug. A high fixed cost for a pharmaceutical innovator can be easily absorbed by a CDMO due to fungible nature of their infrastructure.
Risk Mitigation
Outsourcing to CDMOs allows pharmaceutical companies to share the financial and operational risks associated with drug development. This is particularly important given the high failure rates and unpredictable nature of pharmaceutical R&D. By partnering with CDMOs, pharmaceutical firms can mitigate some of these risks, as the CDMO takes on part of the responsibility for development and production.
Conclusion
Traditionally, the dynamics of outsourcing partnerships have been rooted in a hierarchical structure, where CDMOs were viewed as service providers and drug makers as supervisors. However, the paradigm is shifting towards a model of equal partnership. Successful collaborations now hinge on both parties bringing their unique competencies, knowledge, and capabilities to the table, creating a symbiotic and balanced working relationship.
For reasons stated above, the dynamic landscape of the CDMO industry presents intriguing opportunities and challenges, making it a fascinating sector to watch. Some key players in this space worth noting for their innovative approaches are (a) Laurus Labs, (b) Syngene International, (c) Neuland Laboratories and (d) Piramal Pharma.
Already have an account? Log in
Want complete access
to this story?
Register Now For Free!
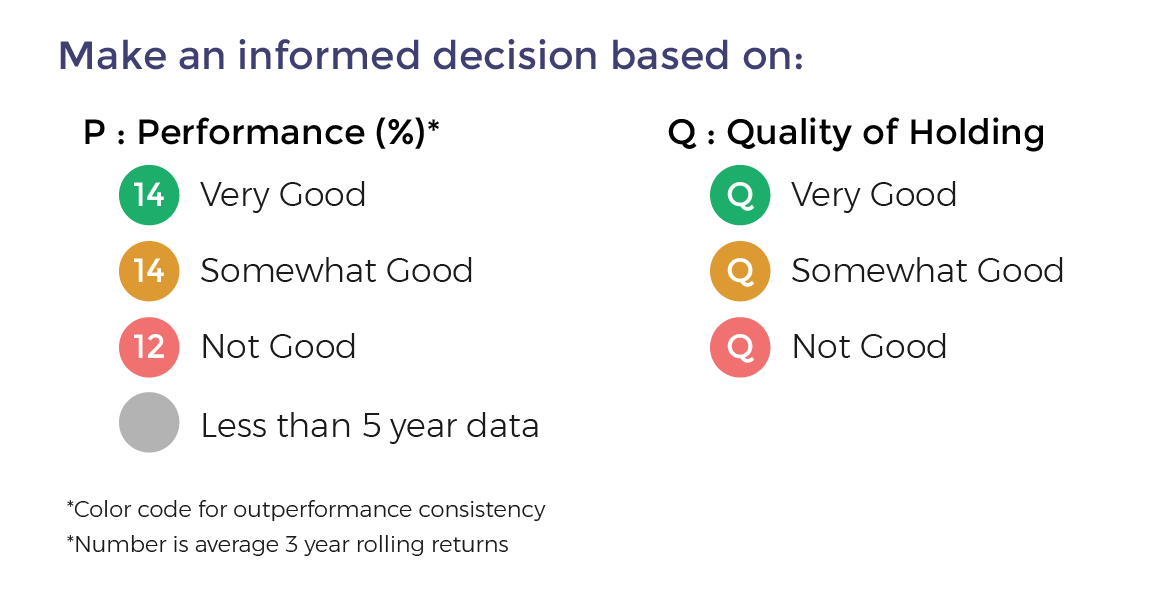
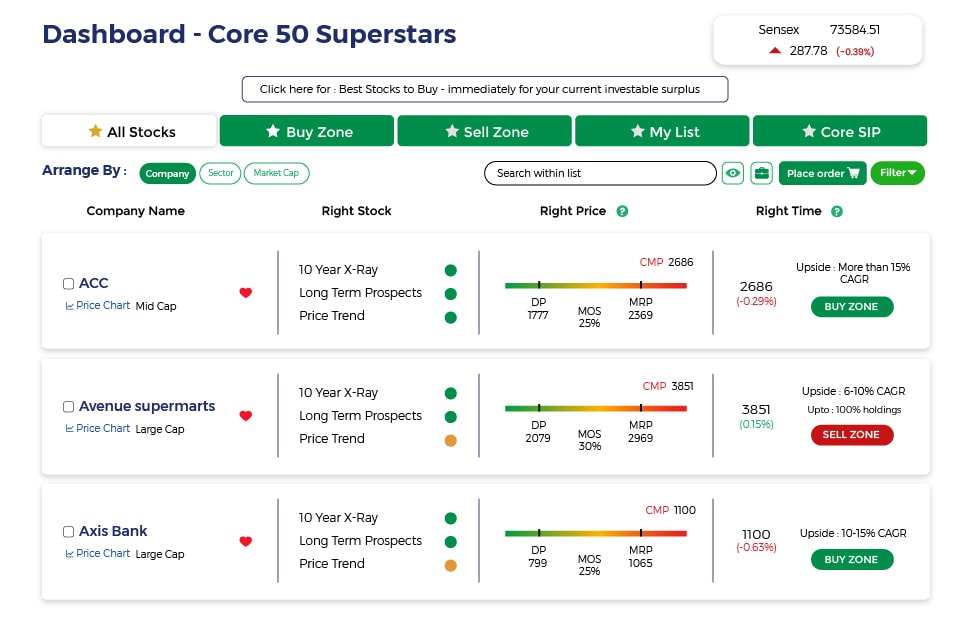
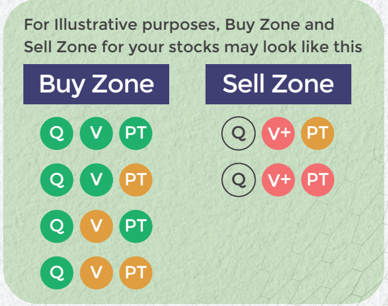
Also get more expert insights, QVPT ratings of 3500+ stocks, Stocks
Screener and much more on Registering.






 Download APP
Download APP

















Comment Your Thoughts: